What Does It Mean if Keq Is Greater Than 1
If Keq is less than 1 it means the concentrations of the reactants are greater than the products and the reverse reaction is favoured. They are related by the equation.

Ch 10 Equilibrium Flashcards Quizlet
When Kc is greater than 1 products exceed reactants at equilibrium.

. If Keq is 1 it means neither forward nor the reverse reaction is favoured. For a general equation like. So aA bB cC dD.
If Keq is much greater than 1 For example if Keq 10 3 then the position of equilibrium is to the right. So Keq 1 favors products Keq 1 favor reactants. If Keq is much smaller than 1 For example if Keq 10-3 then the position of equilibrium is to the left.
When much greater than 1 the reaction goes almost to completion. If the Keq value is less than 1 _____. If Keq is less than 1 it means the concentrations of the reactants are greater than the products.
More reactants are present at equilibrium. Remember the square brackets mean concentration in molarity if it is given in some other unit you must calculate molarity. The position of equilibrium however can change without a change in the value of Kc.
As you can see when K is less than 1 reactants favored deltaG is positive and therefore we say the reaction is not spontaneous. Since the expression of the equilibrium constant depends on the equilibrium concentrations of both the reactans and the products we can. Q Keq Keq is a ratio of k forward over k backward.
More reactants are present at equilibrium. If Keq 1 then the position of equilibrium is in the center the amount of products is roughly equal to the amount of reactants at equilibrium. What does a KC value greater than 1 mean.
More products are present at equilibrium. The concentration of products is greater than the concentration of reactants. When its the oppsite k.
Equilibrium constant Keq is a concise way of stating whether reactants or products are favored in a chemical reaction. Remember that delta G. If Keq is greater than 1 it means the concentrations of the products are greater than those of the reactants.
Also which side of an equilibrium is favored when K is greater than 1. If Keq 1 then the position of equilibrium is in the center the amount of products is roughly equal to the amount of reactants at equilibrium. So if it 1 it means favoring products and it 1 means favoring reactants.
What does Le Chateliers Principle state. Keq Cc Dd Aa Bb. Read more elaboration about it is given here.
If Keq is less than 1 it means the concentrations of the reactants are greater than the products. Products are favored over reactants in the reaction. What does it mean when KC is greater than 1.
WA xB yC zD. Product favored Kq1 means that at equilibrium there are more products than reactants. If the Keq value is greater than 1 _____.
What does the value of KEQ tell you. Its a ratio. Up to 24 cash back aA bB cC dD Keq CcDd.
If Keq 1 then the position of equilibrium is in the center the amount of products is roughly equal to the amount of reactants at equilibrium. If Keq is greater than 1 it means the concentrations of the products are greater than those of the reactants. If Keq is much greater than 1 For example if Keq 103 then the position of equilibrium is to the right.
Keq 1 Keq 1 Keq 1. Keq 1 means that both reactants and products are stable and the reaction is at equilibrium. If Keq is much smaller than 1 For example if Keq 10-3 then the position of equilibrium is to the left.
When Kc is greater than 1 products exceed reactants at equilibrium. If Keq is much smaller than 1 For example if Keq 10-3 then the position of equilibrium is to the left. The equilibrium constant for this reaction can be written as.
Lets take a generic chemical equilibrium to try and determine what an equilibrium constant equal to 1 would mean. When much greater than 1 the reaction goes almost to completion. Since Keq products reactants a large value of k k1 means the reaction will favour the products a lot more meaning when the reaction reached equilibrium you will have mostly products.
What does it mean if Keq 1 at equilibrium. The rate of the forward reaction is greater than the rate of the reverse reaction. The concentration of reactants is greater than the concentration of products.
Keq is equilibrium constant. The Keq is a ratio of reactants and products. Means products reactants Forward rxn is favoured products reactants reverse rxn is favoured products reactants.
If Keq is very large the concentration of the products is much greater than the concentration of the reactants. The equilibrium constant Kc is a constant which represents how far the reaction will proceed at a given temperature. When Kc is less than 1 reactants exceed products.
The Keq equation is written as a mathematical constant being equal to the product of the concentration of the. Coefficients in the chemical equation become exponents in the Keq expression. If delta G is negative then K Upper case K as in Keq or the equilibrium constant will be greater than 1.
Pure liquids solids are not included in the rate expression because their effective concentrations do not change. When K is greater than 1 products favored G is negative and we say the reaction is spontaneous. More products are present at equilibrium.
What you have to understand is that forwardback reaction rates change depending on how much productsreactants are present Le Chateliers principle. What can the value of Keq tell us about a reaction. Keq just tells you what will be favoured at equilibrium.
Chemical equilibrium free energy. Click to see full answer. More reactants are present at equilibrium.

Equilibrium Sch4u Organic Photochromic Molecules Respond To The Uv Light Ppt Video Online Download

Equilibrium Constant Keq Ppt Download

Unit 4 Chemical Equilibrium Keq Ppt Download

Chapter 14 Chemical Equilibrium 14 1 Equilibrium Constant K Eq Objective 1 To Write The Equilibrium Constant Expression For A Chemical Reaction Ppt Download

Chemistry Life The Universe And Everything

Equilibrium Equilibrium Constant K Values The Equilibrium Constant Keq Is A Number Showing The Relationship Between The Concentration Of The Products Ppt Download

9 What Does It Mean If Keq 1 At Equilibrium The Concentration Of Reactants Is Homeworklib
Equilibrium Constants Ppt Download

Solved For Each Equilibrium Constant Indicate If You Would Expect An Equilibrium Reaction Mixture To Be Dominated By Reactants Or By Products Or To Contain Significant Amounts Of Both A K E Q 5 2 Times
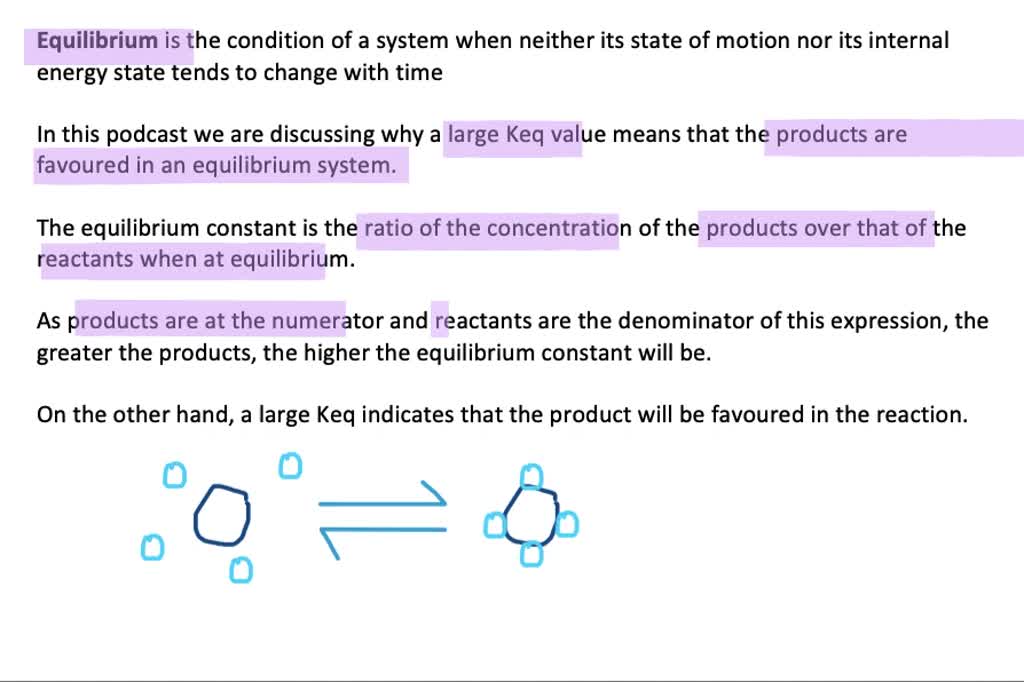
Solved Why Does A Numerically Large Keq Mean That The Products Are Favored In An Equilibrium System

Kaplan Says If Eq Constant Is Then The E Cell Is Positive If Eq Constant Is Then E Cell Cell Is Negative I M Not Understanding The First Two Here They

Chapter 12 Reaction Rates And Chemical Equilibrium Ppt Video Online Download

How To Interpret Thermodynamics Of Reactions Organic Chemistry Help
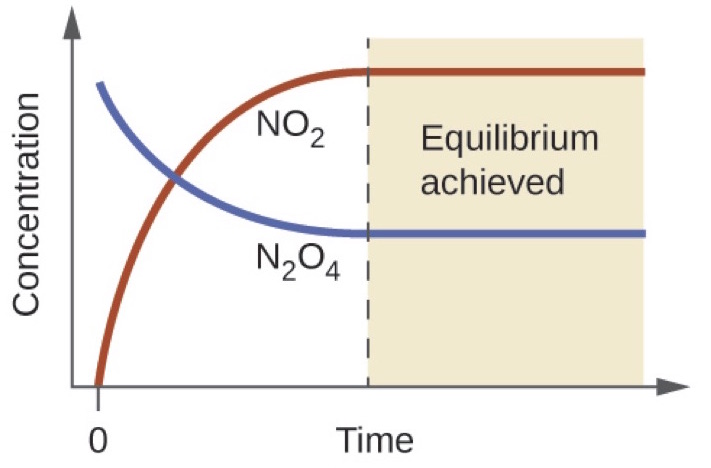
The Equilibrium Constant K Article Khan Academy

Reaction Rates And Equilibrium Ppt Video Online Download
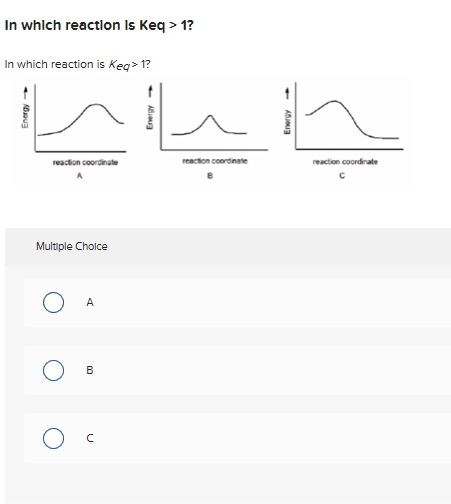
Solved In Which Reaction Is Keq 1 In Which Reaction Is Chegg Com
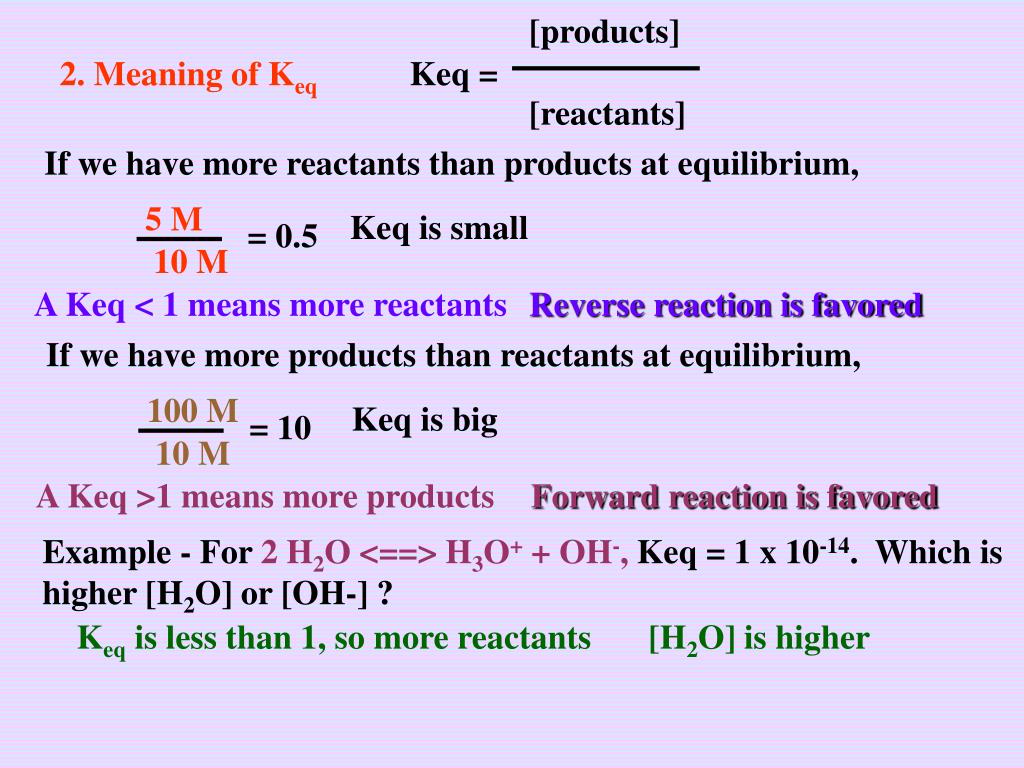
Ppt C Law Of Chemical Equilibrium Powerpoint Presentation Free Download Id 3741786

Comments
Post a Comment